- Cadila Pharmaceuticals Ltd. is one of the largest privately-held pharmaceutical companies in India. Over the past seven decades, we have been developing and manufacturing affordable medicines for patients around the world.
- Our innovation-led drug discovery processes ensure the health and well-being of people around the world. Our enhanced investment in innovation and a strong track record in research and development have produced medical miracles that have changed lives and made a profound impact on real life.
- Being a care-focused, research-driven company, we are committed to complying with the highest ethical standard in clinical research and medical practice.
- We lead our industry in demonstrating the application of cutting-edge research to ethical business practices in producing the alchemy of optimum health outcomes for all.
- Cadila believes in forming strong partnership with ethical pharmaceutical companies to foster mutual growth and enhance market positioning.
Cadila Pharmaceuticals at a Glance

– Shri Indravadan A. Modi
Founder Chairman
“True celebration of research lies as much in inventions as in making the medicine affordable to the last man in the society.”
State-of-the-art Facilities
Regulatory Accreditations

Core Capabilities of Cadila for Partnering CDMO & CMO Business Models
- Comprehensive solutions of CDMO-CMO for Formulation ( OSD, Injectable and others) and Active Pharma Ingredients
- End-to-End Support: Providing comprehensive support from drug development (preclinical or clinical phases) through to commercial manufacturing.
- Efficient Development and Scalable Production: Helping Partner company to optimize time-to-market by providing specialized expertise in formulation, process development, scale-up, and optimization.
- Innovation in Manufacturing: Facilitating to leverage advanced technologies in drug production, including the development of new manufacturing processes or new drug forms ( biologics, complex generics & APIs).
- Cost Efficiency : Offering most cost effective solutions for both development and manufacturing, allowing Partner to avoid building in-house capabilities.
- Regulatory Expertise: Ensuring compliance with regulatory standards (USFDA, EU- Annex-1, MHRA, EMA, WHO & all regulatory bodies) guiding partners through regulatory approval processes for new drugs, APIs formulations.
- Flexibility and Scalability: Providing scalable solutions for Partners. State-of-art digitally integrated Industry 4.0 compliant facility, communicating on IIOT platform with excellent data analytics & AI enabled systems.
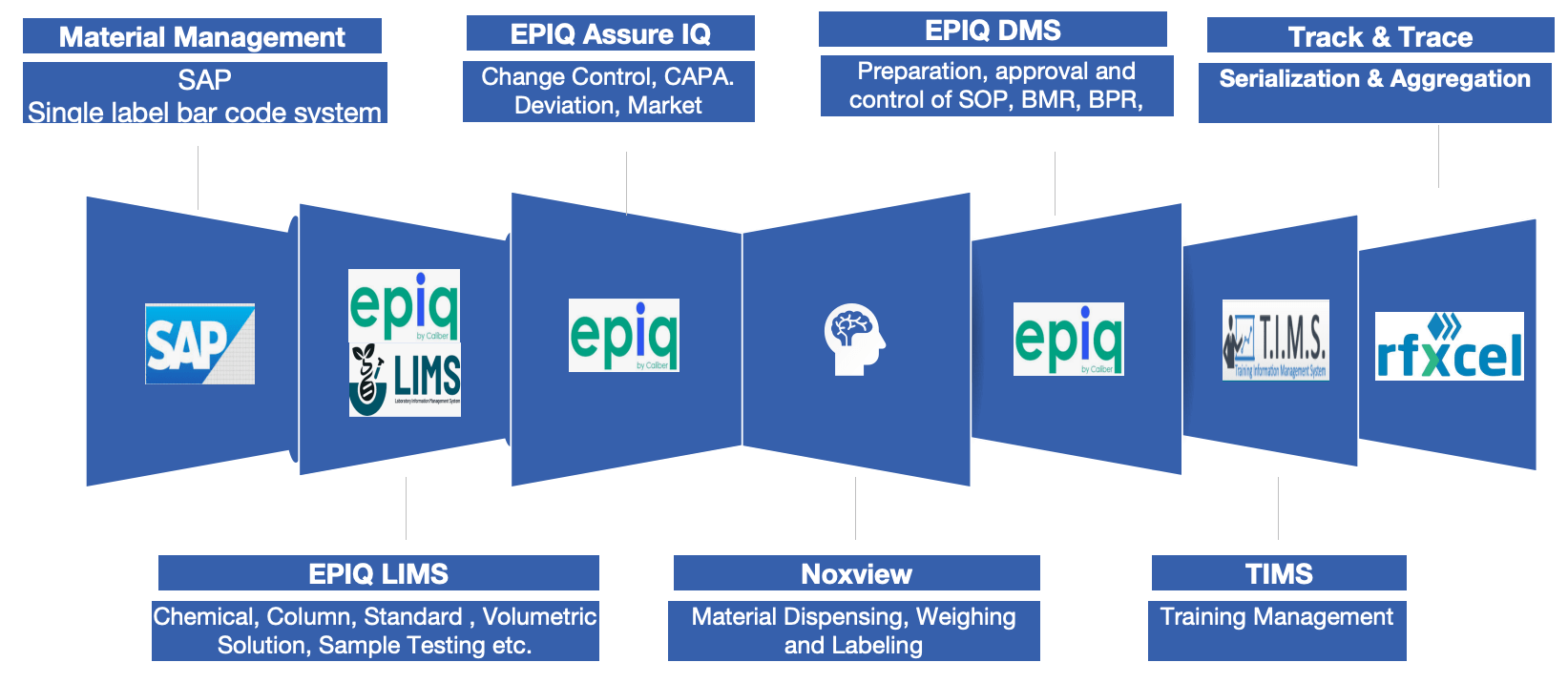
Cadila’s CDMO Project Management process
| Process | Scope |
|---|---|
| Project Alignment: | Coordinating multiple CDMO projects (e.g., Research, Process development, Analytical method validation, Scale-up) to ensure alignment with drug (Substance & Product) development strategy and timelines. |
| Regulatory Compliance: | Maintaining adherence to all relevant regulatory guidelines (USFDA, EMA, EDQM, ICH) throughout the drug development process, including documentation and quality control procedures. |
| Risk Management: | Identifying potential risks associated with manufacturing processes, raw materials, and analytical methods, and implementing mitigation strategies to minimize their impact. |
| Resource Management: | Effectively allocating personnel, equipment, and budget across different CDMO projects to optimize efficiency, Cost effectiveness and meet deadlines. |
| Quality Assurance: | Implementing robust quality systems to ensure the consistent production of high-quality drug Substance and products, including thorough validation and quality control procedures |
| Developing program plans: | Creating a comprehensive program roadmap outlining key milestones, timelines, deliverables, and resource requirements for each CDMO project. |
| Stakeholder management: | Communicating effectively with cross-functional teams (R&D, regulatory affairs, quality assurance) to ensure alignment and address potential issues. |
| Progress monitoring: | Tracking project progress against the program plan, identifying potential delays, and taking corrective actions as needed |
| Change management: | Managing changes to the program scope or requirements, ensuring proper documentation and communication with all stakeholders |
| Reporting and analysis: | Providing regular updates on program status, key metrics, and performance indicators to senior management. |
Research Capabilities
| Formulation Research | Pre-Clinical Research | Clinical Research | API Research |
Complex generics
|
OECD-GLP certified facility | USFDA/ EU/PICs/WHO compliant facility | Robust, scalable and first time right process development |
| New FDCs/Route of Administration/Dosage Forms | Faster Screening of Potential Molecules | Clinical unit facilities in campus (total of 66 beds) | Green chemistry and engineering principles |
| NMEs/NCEs formulations | Molecule specific Animal Model Development for Proof of Concept Studies – 20 therapy areas | Phase I/II/III clinical Trials
|
Quantification & Control of Nitrosoamines & NDSRIs |
| Vaccines/Biosimilars | Toxicity Studies for Regulatory Submissions
|
Bioequivalence & Bioavailablity Studies
|
Solid state chemistry/engineering for identification & quantification of Polymorphic forms |
| – | – |
|
Techniques to meet customer specific requirements e.g. particle size, impurities |
Formulation Research
| Initiation | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|
|
|
|
|
Process Flow : Project Specific Deliverables and Timeline
| IPM / Literature Search | Pre – Formulations | Scale-Up Batches | Process Engineering Batch | Exhibit/Validation Batch | Stability / BioStudies | Marketing Authorization Application |
Pre-Clinical Research
- OECD (Organization for Economic Co-operation and Development) GLP certified facility for integrated Basic & Regulatory Research
- Basic research
- Fast track screening of molecules
- Efficacy studies using animal models for lead compounds
- ADME (PK & other studies)
- Regulatory Toxicity Studies
- Acute
- Sub acute
- Sub chronic
- Chronic
- Male fertility
- Skin sensitisation
- Mutagenicity
- AMES test (bacterial reverse mutation test)
- Chromosomal aberration test (in vivo)
- Micronucleus test (in vivo)
| Studies Conducted | Nos. |
| Acute | 401 |
| Sub acute | 238 |
| Subchronic | 10 |
| Chronic | 3 |
| Male fertility | 5 |
| GPMT | 9 |
| Mutagenicity | 21 |
| Analytical method validation | 5 |
| Total | 692 |
Clinical Research
- Phase I to Phase III clinical trial
- More than 30 clinical trials in more than 10 therapeutic areas
- Bioequivalence / Bioavailability Studies
- USFDA, WHO, EU, PICs country compliant facility
- Efficacy studies using animal models for lead compoundsMore than 400 studies accepted by regulatory authorities of US, EU, PICs and other countries
- 2 Clinical units with 66 beds.
- Fasting & Fed, Special population studies, different administration routes, Single & Multiple dose studies
- Bioanalytical Method Development Capabilities
- Development and validation in different matrix (plasma, serum, urine, whole blood and animal tissues)
- Capacity to store 100 K samples at (-70 ±10 °C ) with dedicated deep freezers
- Capacity to analyse 15,000+ samples per month
- Dedicated pharmacy for investigational product management
- Data management and statistical analysis
- Dedicated Quality Assurance for independent audits of clinical trial sites
Capabilities : Regulatory Affairs
- Navigate through complex network of regulations in various countries
- Complete and first time right submissions to reduce time taken for a product to reach the market
- Strategize implementation of variations/changes to increase efficiency of processes
- Experience in electronic submissions across globe
- US INDs
- Scientific Advice Briefing Packages
- Orphan drug /breakthrough therapy applications
- Global Clinical Trial Applications
- Marketing Authorization Applications
- Drug Master Files/ Certificates of Suitability
- Drug Product approvals in more than 100 countries worldwide.
| World-wide Marketing Authorizations for our Drug Products | |
| USA | 34 |
| Canada & Mexico | 3 |
| Canada & Mexico | 175 |
| Africa | 834 |
| Russia & other CIS countries | 178 |
| South East Asia (SEA) | 297 |
| Middle East | 100 |
| UK/EU/ANZ/WHO | 109 |
| US INDs | 6 |
| Drug master file submitted for our Drug Substances | |
| USA | 32 |
| EDQM (CEP) | 16 |
| EU | 5 |
| Korea | 10 |
| Canada | 13 |
| Australia | 8 |
| Japan | 15 |
| Russia | 3 |
| China | 4 |
| Taiwan | 20 |
| Brazil | 13 |
| Other Countries | 10 |
Our First-In-The-World Innovations
Formulation Manufacturing Operations & Scalability
| Suit No. | Scale of Operation | Granulation | Compression | Coating | Capsule | Inspection | Packaging |
| Tablet/Capsul | Capacity | Tab Capacity per shift in Nos. | Pan Capacity/ Capacity in Kg | Capsule Capacity per Hour | Capsule Capacity per Hour | Capacity | |
| 1 | 10,000 to 100,000 | 50 L | 400,000 | 24″ – (7 – 14 kg) | 90,000 | 1,00,000 Tab | 80 Blister/Min |
| – | 175,000 | – | – | – | 100 Strip/Min | ||
| 2 | 100,000 to 250,000 | 100L | 600,000 | 43″ (30-90 kg) | – | 1,00,000 Tab | 120 Blister/Min |
| – | 225,000 | 53″ (70-150 kg) | – | 1,00,000 Cap | 100 Strip/Min | ||
| – | 400,000 | – | – | 100 Strip/Min | |||
| – | 400,000 | – | – | – | |||
| 3 | 250,000 to 1,000,000 | 400L | 1,850,000 | 70″ (200-450 kg) | 2,00,000 | 3,00,000 Tab | 120 Blister/Min |
| 300 L – Wruster coating | 1,600,000 | 70″ (200-450 kg) | – | 120 Blister/Min | |||
| – | 1,200,000 | – | – | 60 Blister/Min | |||
| 120 Strip/Min | |||||||
| 4 | 1,000,000 to 2,500,000 | 600 L | 3,000,000 | 53″ (70-150 kg) | 2,00,000 | 3,00,000 Tab | 120 Blister/Min |
| – | 1,850,000 | 70″ (200-450 kg) | – | 120 Blister/Min | |||
| 120 Strip/Min | |||||||
| 5 | 2,500,000 to 4,500,000 | 1200 L | 3,000,000 | 70″ (200-450 kg) | 2,00,000 | 6,00,000 Tab | 360 Blister/Min |
| 2500 L – Dry Mixing | 1,900,000 | 70″ (200-450 kg) | – | – | 120 Bottle/Min | ||
| – | 2,500,000 | – | – | – | 120 Blister/Min | ||
| – | – | – | – | – | 300 Strip/Min – 3 Machines | ||
| – | – | – | – | – | 240 Strip/Min | ||
| – | – | – | – | – | 120 Strip/Min |
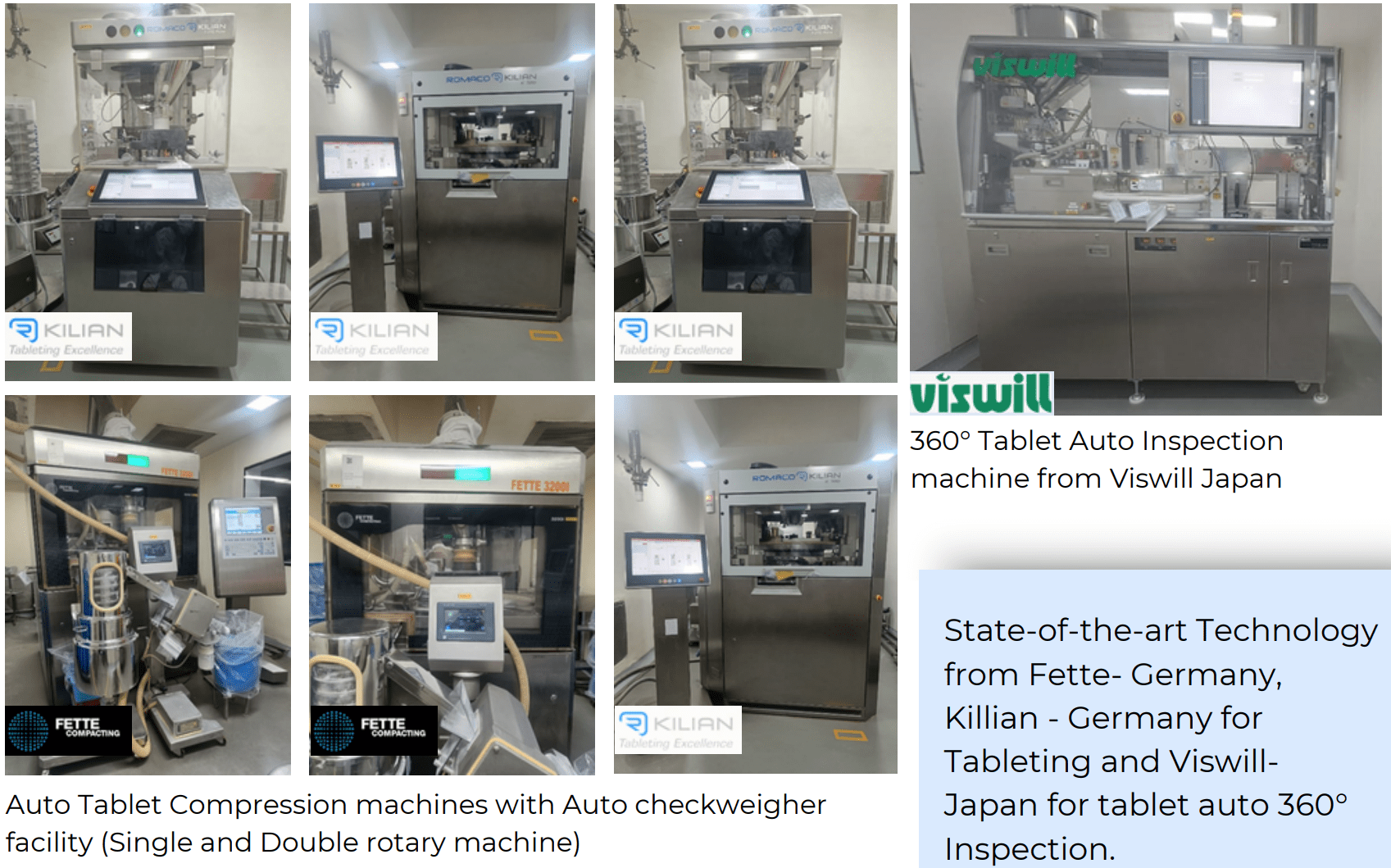
| Area | OEM |
| Granulation | Glatt |
| Compression | Fette /Killian – Germany |
| Coating | Glatt |
| Inspection | Viswill – Japan |
| Primary Packing | CVC – Taiwan, CAM – Italy |
| Secondary packing | CAM – Italy, ACG |
Note: All machines are Industry 4.0 compliant, communicating on IIOT platforms with excellent data analytics & AI enabled systems.
Manufacturing Equipment & Capacity (Gujarat)
Manufacturing Capacity (Oral Solid Dosage)
| Dosage | Annual Capacity |
| Tablets | 15 Billion |
| Capsules | 1 Billion |
| Area | Lines |
| Granulation | 7 lines |
| Compression | 14 lines |
| Coating | 9 lines |
| Visual Inspection | 3 lines |
| Capsulation | 2 lines |
| Packing | 18 lines |
Manufacturing Capacity (Injectable)
| Dosage | Annual Capacity |
| Ampoule | 45 Million |
| Vial | 65 Million |
| Lyophilized | 2.5 Million |
| PFS | 10 Million |
| rDNA ( Vials) | 15 Million |
| Area | Lines |
| Ampoule | 1 line |
| Vial Filling | 3 lines |
| Packing | 3 lines |
| rDNA | 1 line |
Manufacturing Technologies
Vaccines and Biosimilar Capabilities

Capabilities for R&D and Manufacturing
Vaccines (200L Production scale)
-
- Rabies vaccine (Commercial)
- Influenza Vaccine (Commercial)
- HPV Vaccine (Clinical Development)
- Chicken Pox Vaccine (Clinical Development)
Biologics (1500L Production scale)
-
- STPase -Streptokinase
- CADICRIT – Erythropoietin
- FILCAD -GCSF
- Sepsivac – Mycobacterium w
- MYCIDAC C – Mycobacterium w
Current CDMO Projects
Neuromuscular blocker : Botulinum Toxin – ATGC ,South Korea
-
- ATGC received MAA (Marketing authorization) received
Antiplatelet Anticoagulant (APAC) – Aplagon ,Finland
-
- Phase I Clinical trial supplies in Europe
Novel Vaccine Development Program
Select proteins important for inducing neutralizing antibody and CMI (Cell mediated immunity)
- Surface hemagglutinin (HA)
- Neuraminidase (NA)
- Matrix (M1)
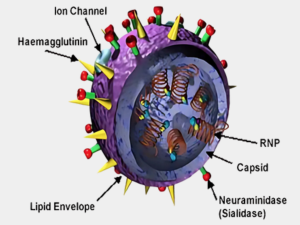
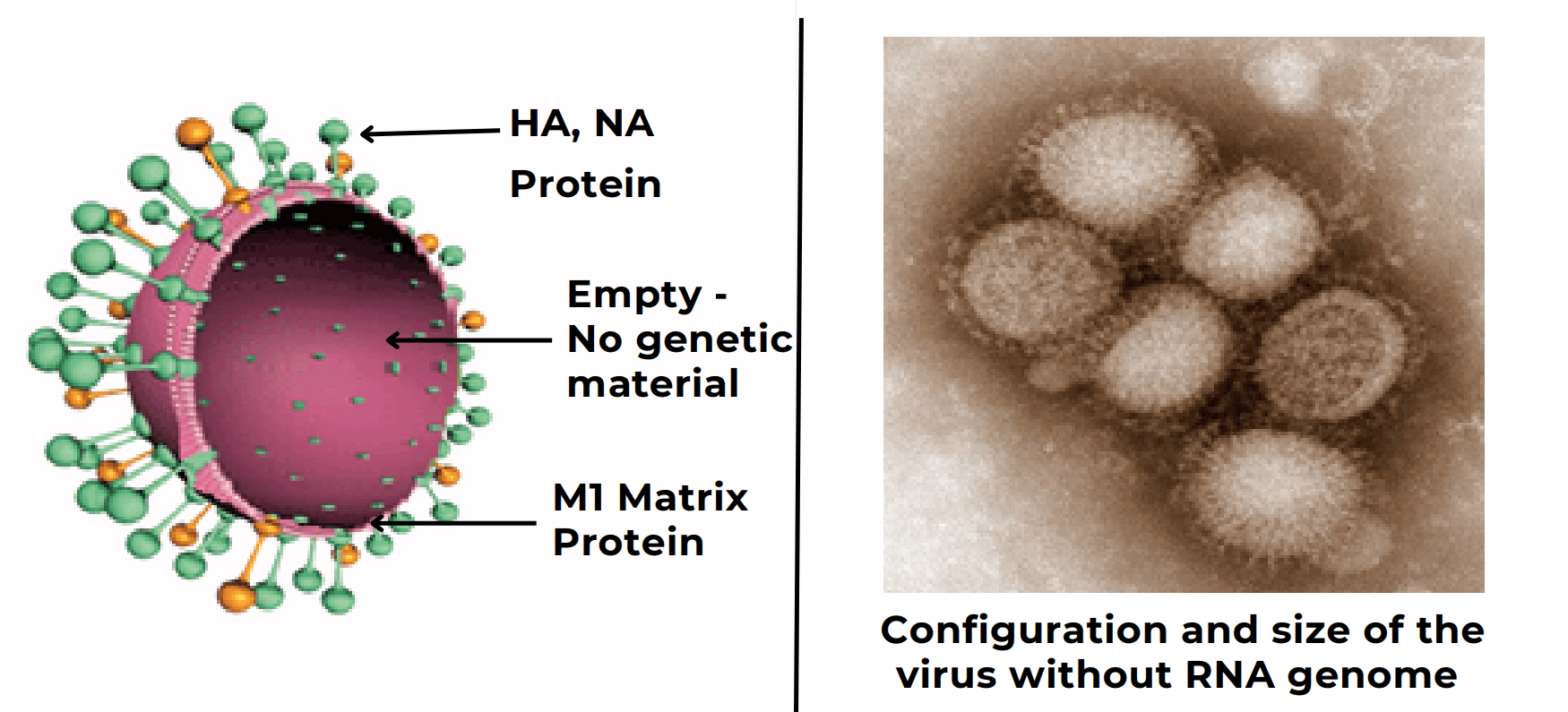
Virus-Like Particles (VLP) Seasonal & Pandemic Influenza
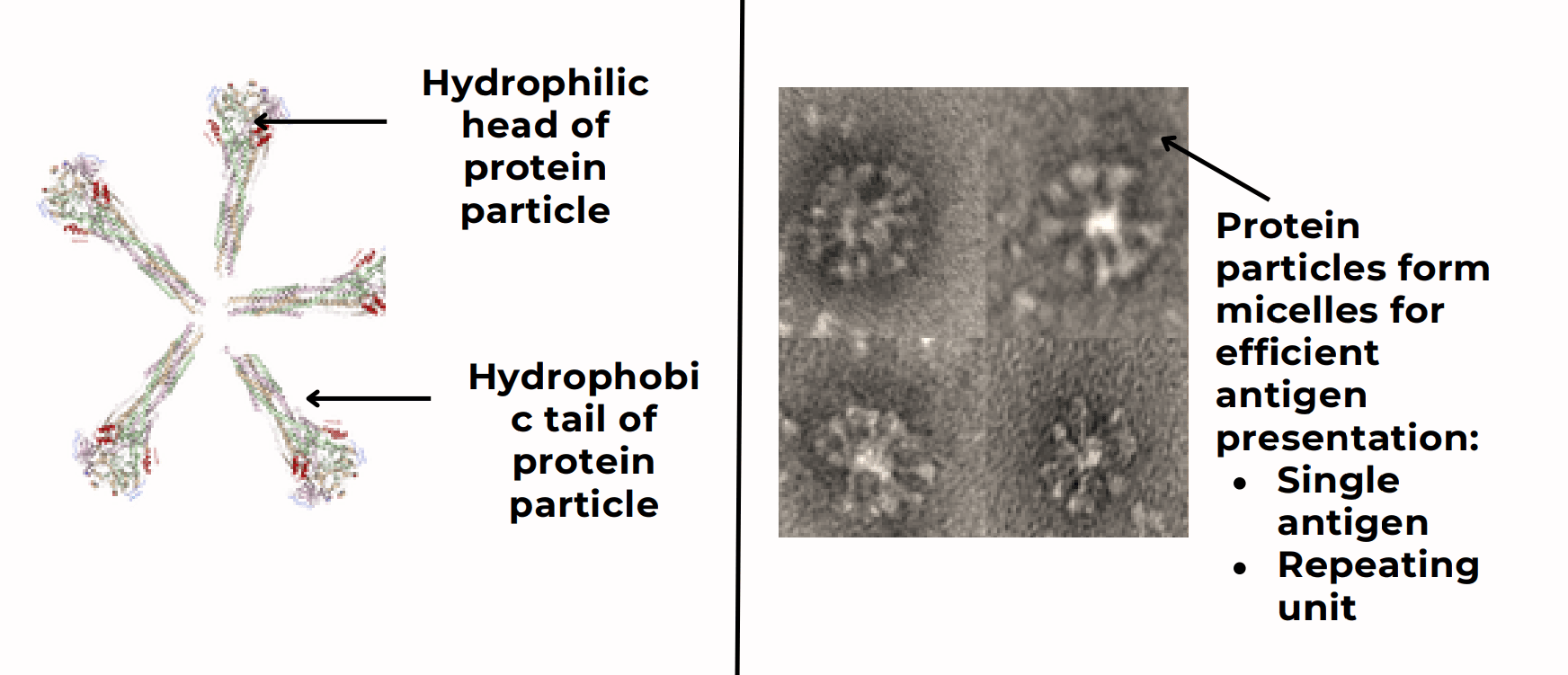
Recombinant Protein Micelles Rabies
VLP Vaccine Manufacturing Process flow
- Experience of developing the process from R&D scale to 10L Wave Bioreactor and Stirred Bioreactor using the Single use technology.
- Process demonstrated to scale up at 100L Wave Bioreactor and further scale up in 200L Single Use Stirred Bioreactor.
- The GMP manufacturing process is based on Single-use technology for faster product development and market supply.
- Rich experience of engineering and design of Drug Substance and Drug Product facility upto 2000L scale (Executed Detailed Engineering).
Scalable and stable manufacturing technology

Vaccine and Biosimilar Facility
Overview of State-of-the-art Injectable plant (Greenfield-2024)
| Aseptic Filling | Target Market |
| Aseptic filling of general category injectable products, Vial Filling (Lyophilized) and liquid | US , Europe, ANZ, Emerging Markets. |
| Product Category | Container size / capacity | New Plant Volumes |
| Vials Filling | 5,10 ml Vials Other sizes can be filled | Filling rated capacity : 20 Mio units / Annum/ 1 shift basis 40 Mio units / Annum /2 shift basis |
| Vial Lyphlization | 20 M2 (38000V/Load) x 2 no | Lyo two numbers rated capacity: 11.4 Mio/Annum |
Injectable Plant and Automation
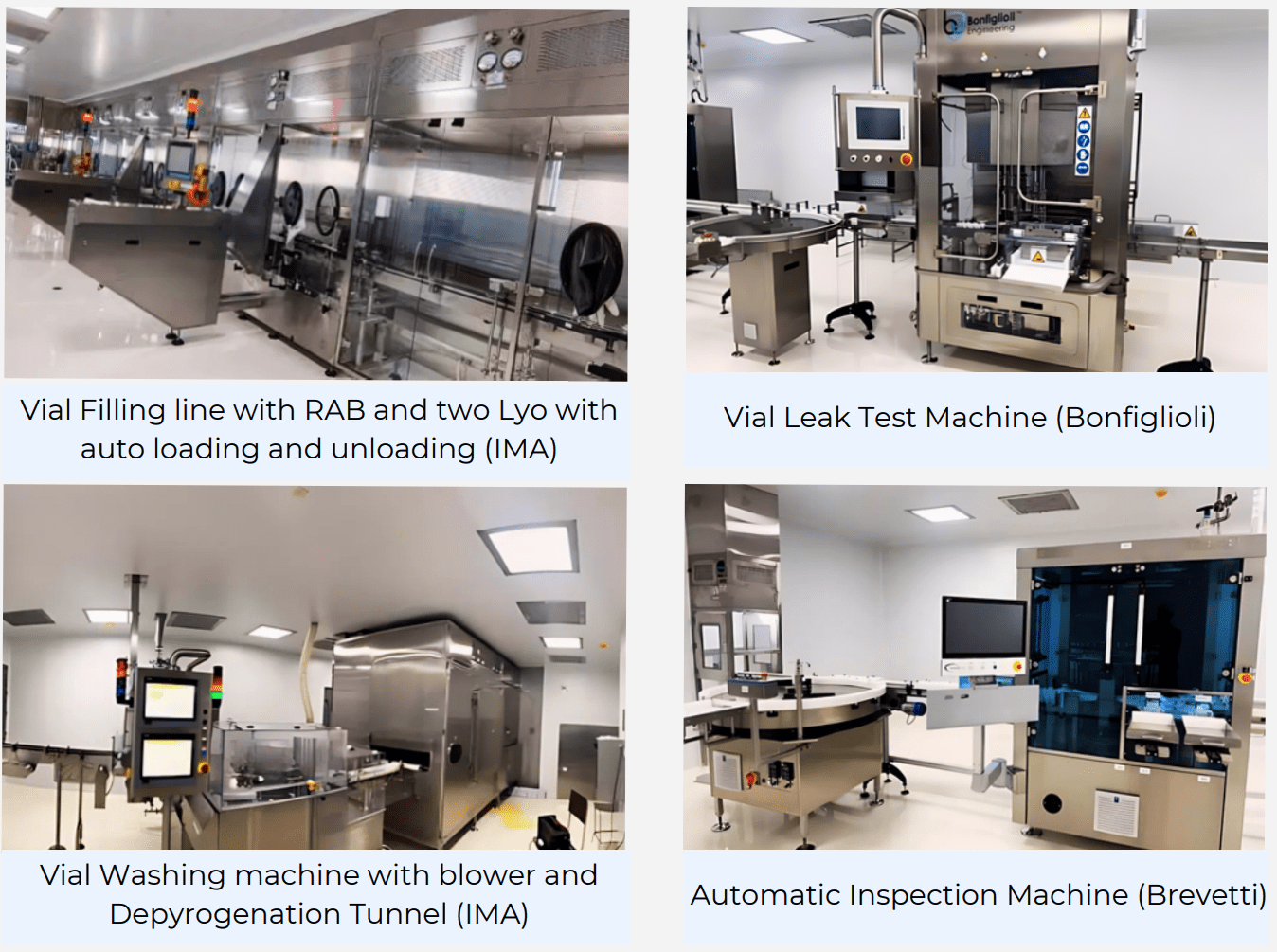
| Sr. No. | Room Name | Equipment | Qty | Speed / Capacity | Vendors |
| 1 | Washing and sterilization | Vial Washing machine with blower | 1 | 200VPM @10ml | IMA |
| Depyrogenation Tunnel | 1 | 200VPM @10ml | IMA | ||
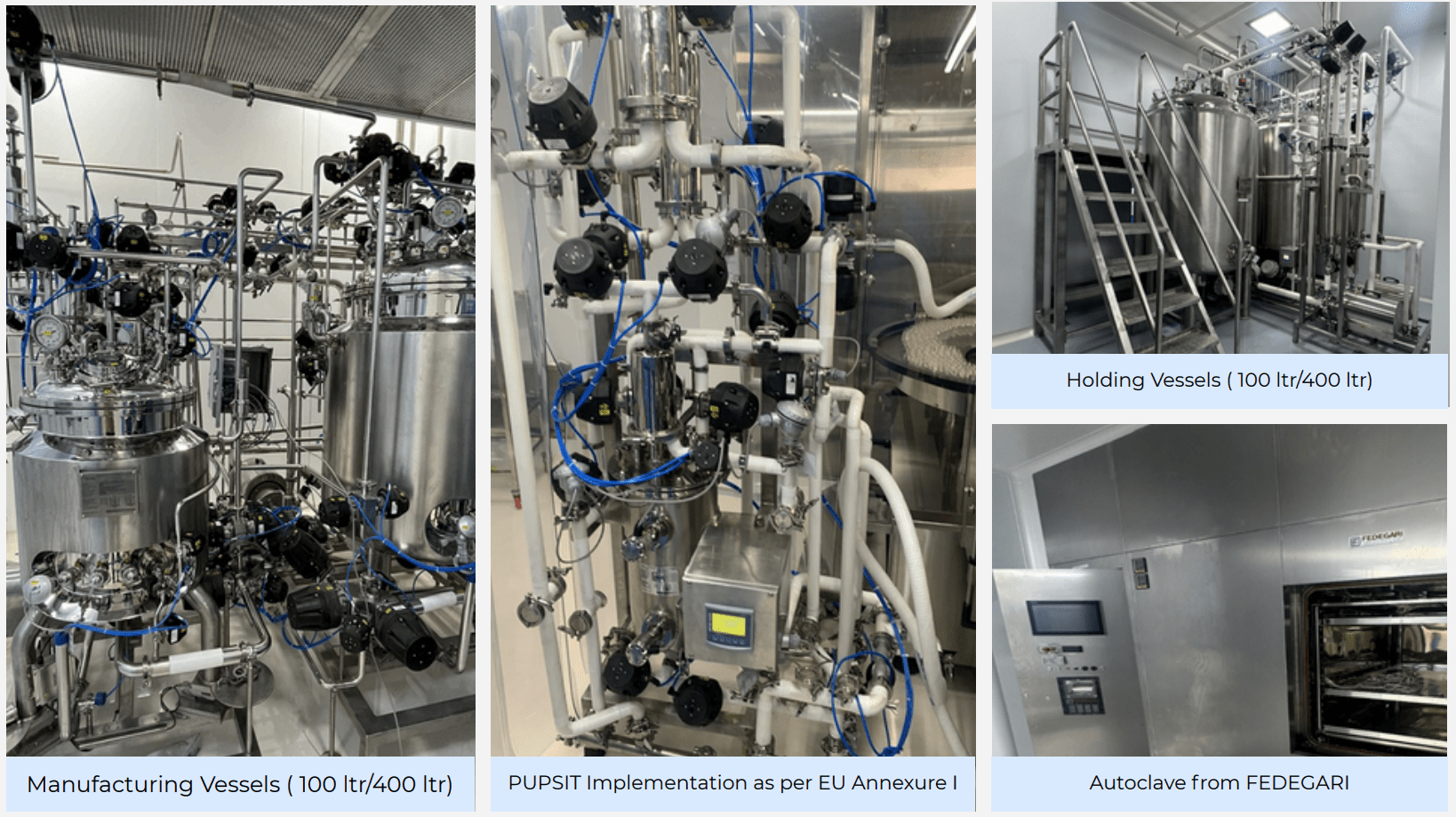
| 2 | Compounding | Manufacturing Vessel | 2 | 100 Ltrs/400 Ltrs | BioAspire |
| 3 | CIP / SIP Mfg. | CIP/SIP SKID | 1 | 100 Ltrs/400 Ltrs | BioAspire |
| 4 | Vial Filtration ,PUPSIT | Filtration Vessel | 1 | Online Filtration | BioAspire |
| 5 | Vial filling | Filling machine | 1 | 200VPM @10ml | IMA |
| Filter Integrity machine | 1 | – | Sartorious | ||
| 6 | LYO | Freeze Dryer | 2 | 38000 vials @10ml | IMA |
| 7 | LYO | CIP/SIP SKIDAuto Loading and Unloading | 1 | 38000 vials @10ml | IMA |
| 8 | Capping room | Capping machine | 1 | 200VPM @10ml | IMA |
| 9 | Component prep and sterilisation room | Steam Sterilizer | 1 | 1200x1200x1200mm | Fedegeri |
| 10 | Leak testing | Vial Leak test machine | 1 | 200VPM @10ml | Bonfiglioli |
| 11 | Optical Inspection | Optical Inspection m/c with Xray and moisture content /leak test | 1 | 200VPM @10ml | Brevetti |
| 12 | Packaging |
|
1 | 200VPM @10ml |
|
| Sr. No. | Name of Equipment/Instrument | Make | SCADA/ Server |
| 1 | WMS for warehouse RM PM | BCI | 1 |
| 2 | Cold Room (RM Area) | Bluestar | 1 |
| 3 | SCADA for Dispensing & Weighing Automation | BCI | 1 |
| 4 | EMS SCADA (Dispensing Booth, sampling Booth, LAF, Mobile LAF trolly, Passbox ) | Siemens | 1 |
| 5 | Autoclave (Horizontal) for equipment and components | Fedegari | 1 |
| 6 | Lyo CIP/SIP Skid and accessories | BioAspire | 1 |
| 7 | Part Washing Machine and accessories | Pharma Lab | 1 |
| 8 | Filter Integrity Tester | Sartorius | 2 |
| 9 | 400L/100 L Manufacturing & Holding tank & filtration Skid and accessories Pupsit | Bio Aspire | 4 |
| 10 | Non-viable particle counter offline for production area (Air Sampler) | Metone | 2 |
| 11 | Glove Leak Tester with accessories | Shreedhar | 1 |
| 12 | Online particle monitoring | PMS Aimil | 1 |
| 13 | E Log Book | BCI | 1 |
| 14 | IMA Vial Washing machine | IMA | 1 |
| 15 | IMA Vial De pyrogenating tunnel | IMA | 1 |
| 16 | Vial Filling Line | IMA | 1 |
| Sr. No. | Name of Equipment/Instrument | Make | SCADA/ Server |
| 17 | Polaris 1 | IMA | 1 |
| 18 | Polaris 2 | IMA | 1 |
| 19 | Lyophilizer 1 | IMA | 1 |
| 20 | Lyophilizer 2 | IMA | 1 |
| 21 | Vial Capping Line | IMA | 1 |
| 22 | Cold Room (FG Area) | Bluestar | 1 |
| 23 | Incubator 20000 litrs | Thermolab Server | 1 |
| 24 | Deep Freezer | Thermolab Server | 1 |
| 25 | Bonfiglioli leak testing machine | Bonfiglioli | 1 |
| 26 | Optical Inspection | Brevetti | 1 |
| 27 | Track and Trace | ACG | 1 |
| 28 | Cartonating Machine | ACG | 1 |
| 29 | Labelling Machine | Ambica | 1 |
| 30 | Check weighing System | Technofour | 1 |
| 31 | WMS for warehouse SPM FG | BCI | 1 |
| Total SCADA | 28 |
Utilities and quality systems
| Sr. No. | Name of Equipment/Instrument | Make | SCADA/ Server |
| 1 | Post Water Treatment System (Potable Water , Ozone Generation System , PW Generation (RO+EDI)+PW Distribution, WFI System) | Praj SCADA | 1 |
| 2 | WFI GENERATION PLANT | Pharma lab SCADA | 1 |
| 3 | PURE STEAM GENERATOR | 1 | |
| 4 | Building Management System BMS | Siemens SCADA | 1 |
| 5 | Environmental Management System EMS | 1 | |
| 6 | ELV (Low Voltage , Voice, Data) | 1 | |
| 7 | Utility Management System , Chiller (four no , Air compressor (two no) and other equipment | Siemens SCADA | 1 |
| Total | 7 |
| Sr. No. | Name of Equipment/Instrument | Make | SCADA/ Server | Back up server/SCADA |
| 1 | FTIR (Fourier Transform Infrared Spectroscopy) | Shimadzu | Shimadzu Server | Back up server and
LIMS Server |
| 2 | TOC analyser | Shimadzu | Shimadzu Server | |
| 3 | GC (Gas chromatography) | Shimadzu | Shimadzu Server | |
| 4 | UV Spectrophotometer | Shimadzu | Shimadzu Server | |
| 5 | Karl Fisher | Karl Fisher | Metrohm Server | |
| 6 | Autotitrator | – | Metrohm Server | |
| 7 | HPLC | Waters | Water Server | |
| 8 | Liquid Particle counter (LPC) | – | SCADA |
| Sr. No. | Name of Equipment/Instrument | Make | SCADA/ Server | Back up server/SCADA |
| 9 | pH meter | Labindia | – | Back up server and
LIMS Server |
| 10 | pH meter | Labindia | – | |
| 11 | Weighing Balance | Sartorius | – | |
| 12 | Stability Chamber | Thermolab | Thermolab Server | Back up server |
| 13 | BOD Incubator | Thermolab | Shimadzu Server | |
| 14 | Deep Freezer | Thermolab | Shimadzu Server | |
| 15 | Cooling chamber | Thermolab | Shimadzu Server | |
| 16 | Air Sampler | Shreedhar | SCADA | |
| 17 | Horizontal Autoclave | MachinFabrik | SCADA |
Data Integration on IIOT Platform
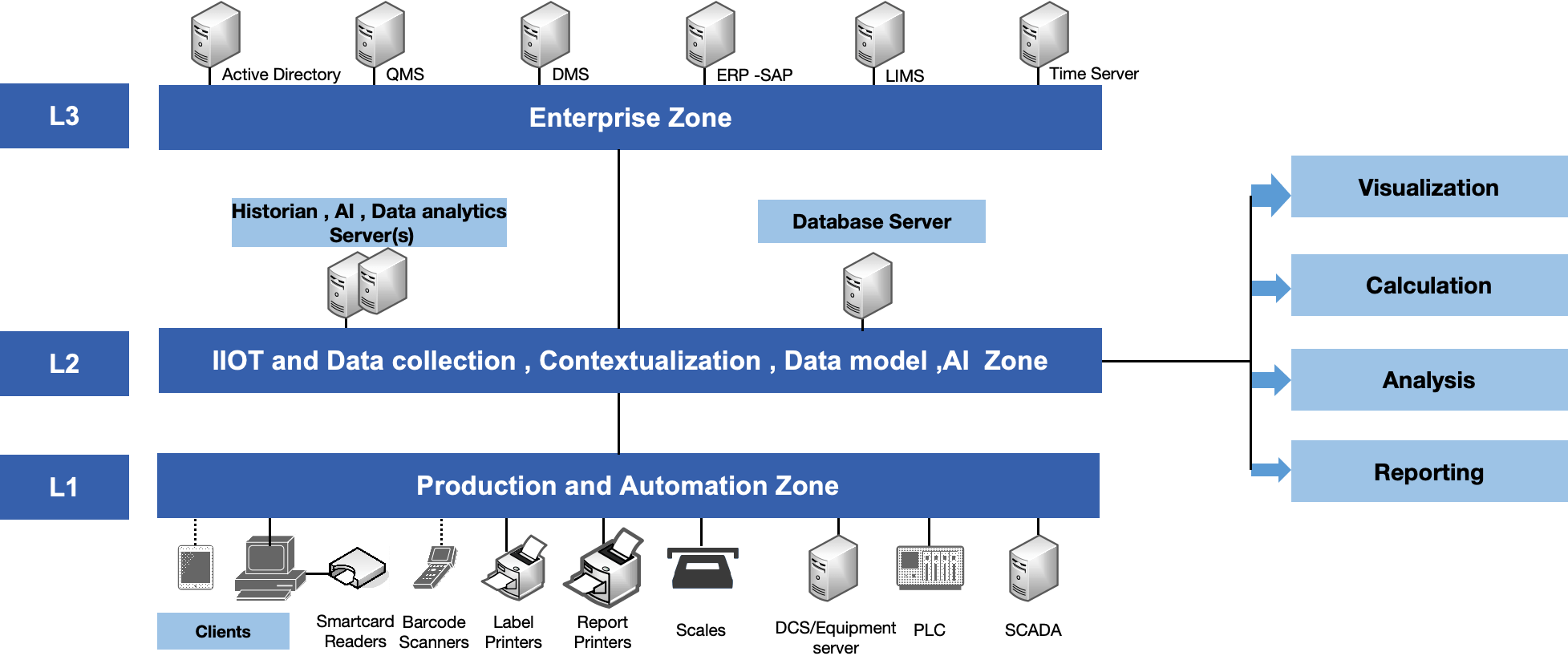
State-of-the-art digitally integrated industry 4.0 complaint facility with excellent data analytics, AI enabled systems.
Our Approach to Faster Development of Technology- Drug Substance (API)
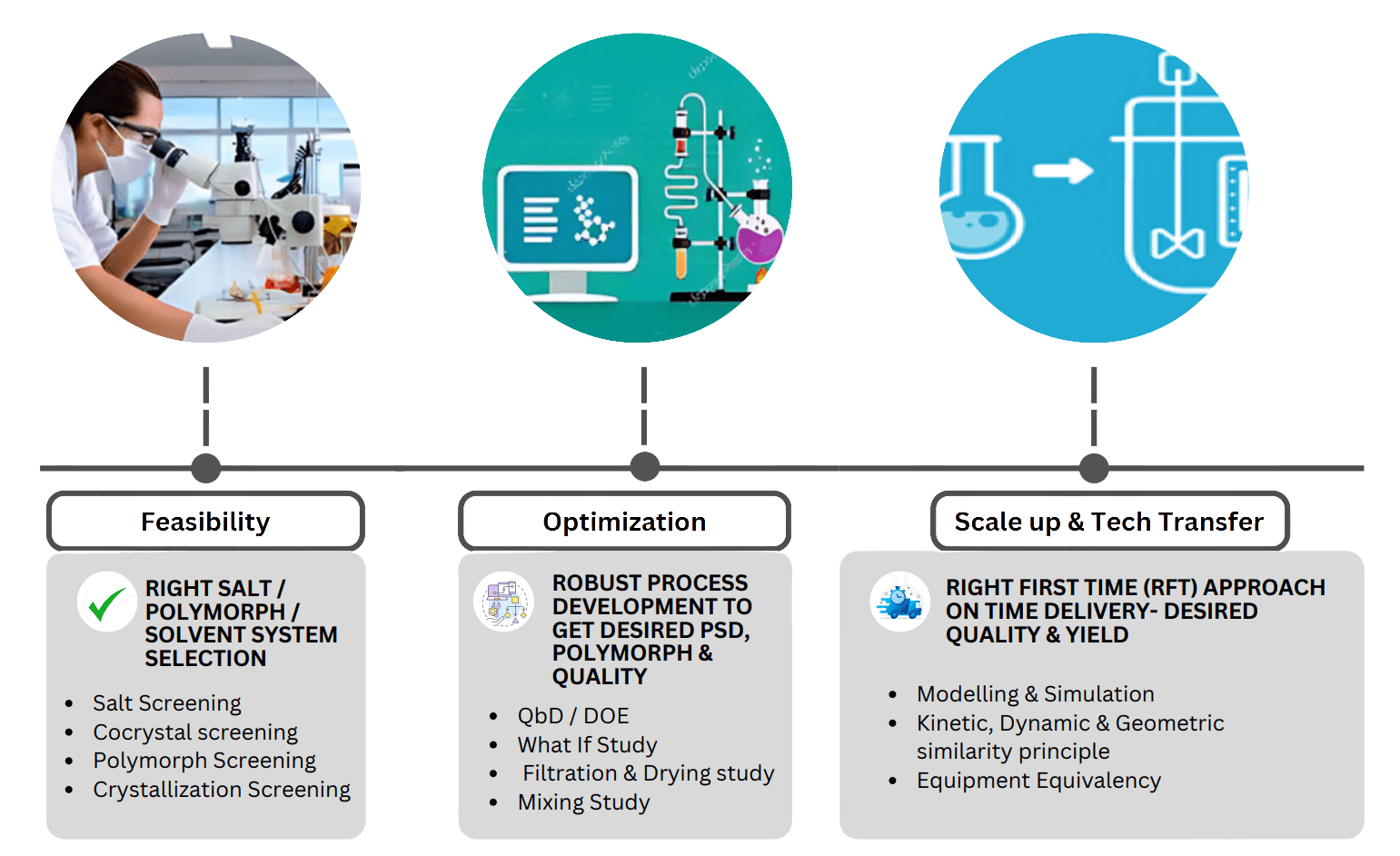
API R & D - Reaction Capabilities & Expertise
- Grignard reaction
- Cryogenic reaction (–850 C)
- High temperature reaction Stereo selective chiral catalysis reactions
- Bromination reaction
- Hantzsch pyridine synthesis
- Friedel craft Alkylation/acylation reaction
- Hydrogenation reaction (with Ni, 10% Pd/C)
- Hydrolysis of esters, decarboxylation, amides and Nitriles
- Condensation Reaction
- Methylation Reaction
- Cyanation
- Hoffman degradation reaction
- Chiral separation/resolution
- Chlorination reaction
- Paal-knorr pyrrole Synthesis
- Mannich reaction
- Fischer Indole Synthesis
- Epoxidation
- Azide chemistry
- Sandmeyer Reaction
- Trans-esterification Reaction
- Handling of HF & BF3
- Optical Resolution
National Collaborations & Partnerships

API Analytical Capabilities




- 300 MHz NMR -1 No. [Bruker]
- LC/MS/MS -1 No. [Applied Bio-systems]
- GC/MASS ( thermo)
- PXRD – 1 No. (Pan Analytical)
- ICPMS (Thermo)
- HPLC- 20 Nos
- UPLC- 2 Nos
- Prep HPLC- 1 No.
- GC (Normal & Space)- 4 Nos
- Differential Scanning Calorimeter (DSC)- 1 No.
- Thermo Gravimetric Analysis (TGA)- 1 No.
- IR Spectrophotometer- 1 No.
- UV Spectrophotometer- 1 No.
- Polarimeter- 1 No.
API Process Engineering Research Lab (PERL)
- Engineering Target Studies
- Kinetics & Thermodynamic Studies
- Process Safety & Reaction Calorimetry
- Mass Transfer & Flow engineering
- Suitability & selection of unit process
- Solid-Liquid Separation
- Drying Technology
- Crystallization & Particle Morphology
- Quality by Design (QbD) or Quality Target Profile
- Process Analytical Technology (PAT Studies)
- Critical Process Parameters (CPP)
- Critical Material Attributes (CMA)
- Critical Quality Attributes (CQA)
- Design of Experiments (DOE Studies)
- Modelling & Simulation Studies



Active Pharmaceutical Ingredients (API) Development Cycle

API Development Timeline

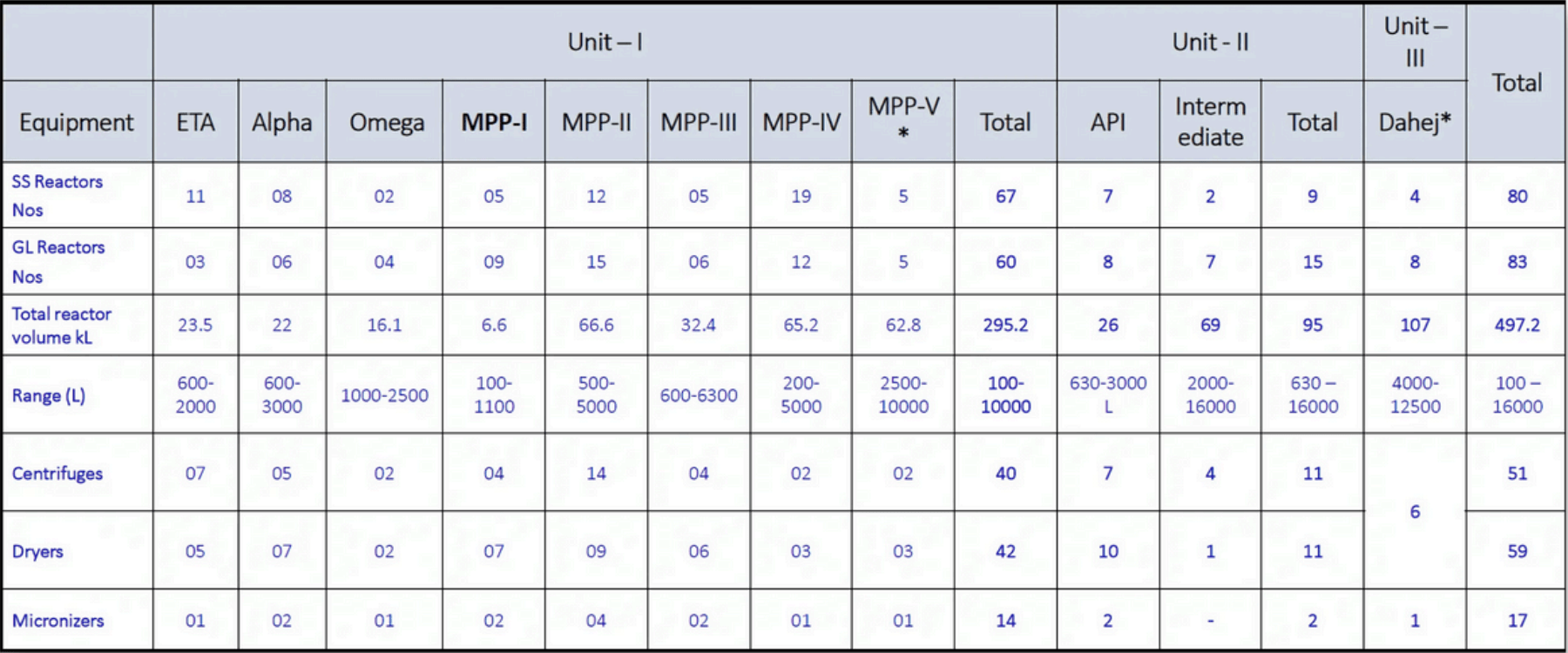
API Manufacturing : Capacity & Capabilities
Our green field state-of-the-art DCS controlled API facility is now operational
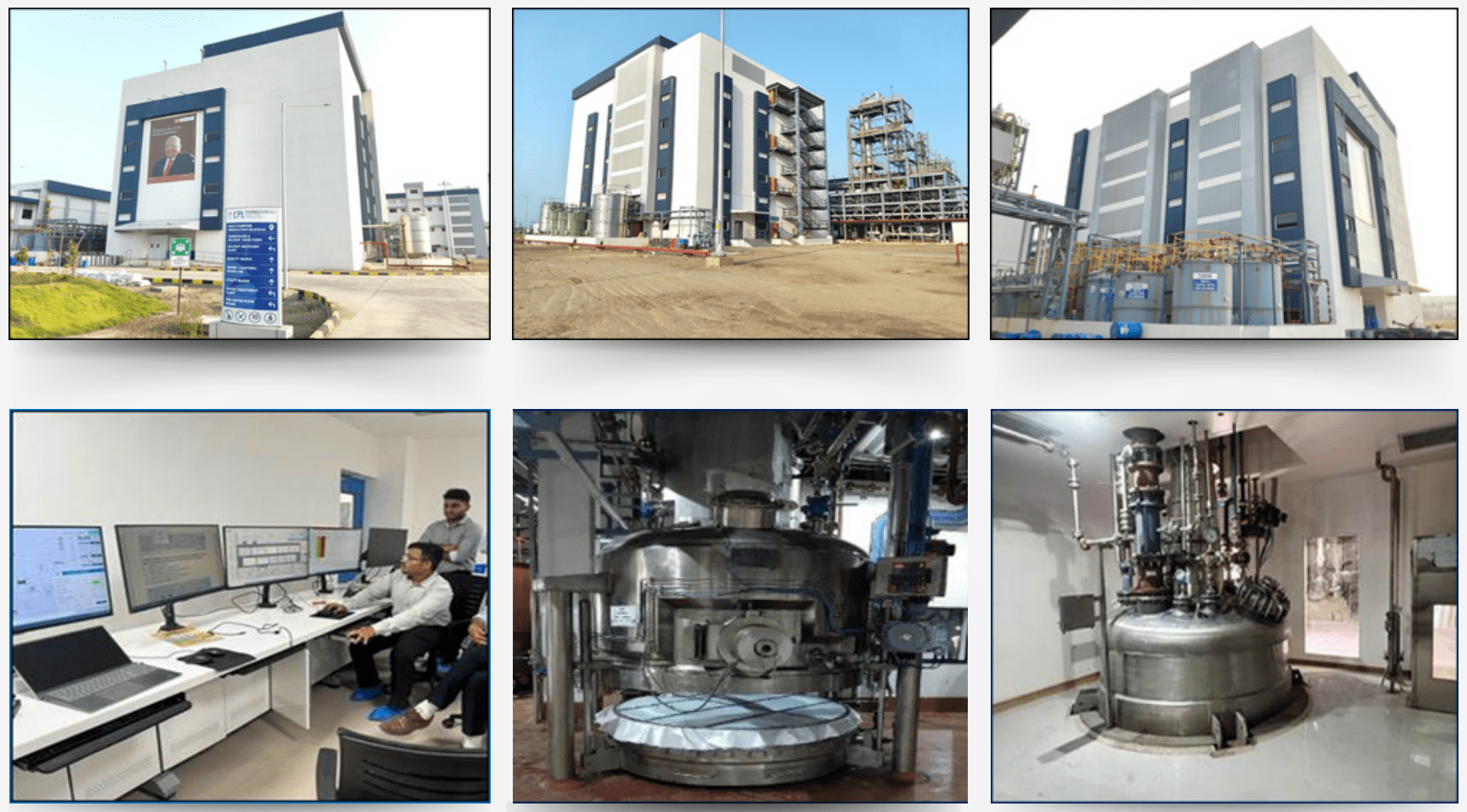
Digitalization in Quality systems and Manufacturing
CDMO Capabilities Powered by our Indigenous Advanced Machineries

Adaptive Kommunikation
-
- Eliminates Hardwires
- User Interference Enhancement
- Multiple Parameters Monitoring
- Easy Diagnosis
- Additional Safety Features
- Increase machine efficiency
- Better man machine experience
- Industry 4.0 adaption .
- Upgradable
Indigenous Advanced Machineries- Engineering Excellence

Next Generation Tablet Press – NXi4
- Double sided Rotary with 10 T main & Pre Compression
- Centre Drive Turret
- Three paddle force feeding system with powder level sensor
- Auto Wt. control & Single tablet rejection with 21CFR part -11 compliance
- Auto Lubrication system & drip feed lubrication for punches
- Industry 4.0 adaption
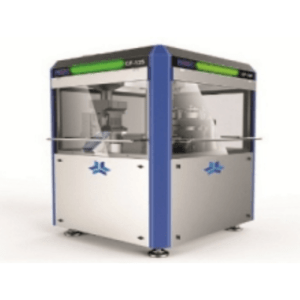
Capsule Filling machine – CF 125
- 125000 Capsules / hr. with all possible combinations of powder, Pellet, Tablet & Micro Tablet.
- Rigid & ergonomically indexer Globoidal Cam design.
- Compact Turret Design with chain driven main drive.
- Capsule body & Cap Segment are single piece
- 21CFR part 11 Compliance.
- Industry 4.0 adaption
Sustainability & ESG Commitment
At Cadila Pharmaceuticals, we are dedicated to sustainable growth through responsible environmental, social, and governance (ESG) practices. We are committed to creating a positive impact through the following key initiatives:
Sustainability Plans & Actions:
- 3-Year ESG Roadmap: Collaborating with BDO India LLP, we have developed a strategic ESG roadmap to drive sustainability across our operations.
- ESG Oversight: A dedicated ESG Committee ensures consistent oversight and reviews of our ESG priorities.
- GHG Emissions Inventorization: We’ve inventoried Scope 1, 2, and 3 GHG emissions, focusing on Scope 3 for our decarbonisation journey.
- Renewable Energy Transition: We’re increasing the share of renewable energy by developing wind and solar hybrid projects.
- Enhanced Sustainability Disclosures: We’re extending our GRI-based indicators and aligning disclosures with international frameworks and consortia (Sustainability Accounting & Standards Board (SASB), Bio Pharma Sustainability Roundtable, Pharma Supply Chain Initiative (PSCI)) including the EU’s Corporate Sustainability Reporting Directive (CSRD).
- Green Building Certification: Cadila is actively pursuing Indian Green Building Council (IGBC) certifications across all its operational facilities
- ESG Data Management: We’ve implemented an advanced ESG Data Management System to digitally track, monitor and inform better decision-making on our non-financial performance.