Cadila Pharma receives licence from MPP for generic version of Pfizer’s Covid-19 oral antiviral – nirmatrelvir
17th Mar, 2022 | NewsNirmatrelvir in combination with low dose ritonavir, for the treatment of mild-to-moderate Covid-19 patients at high risk of progressing to severe illness, has shown significant reduction in hospitalisation and deaths. The drug is approved or under emergency use authorization for COVID-19 in many countries, including the US, UK, EU, Singapore, Australia, Japan and China.
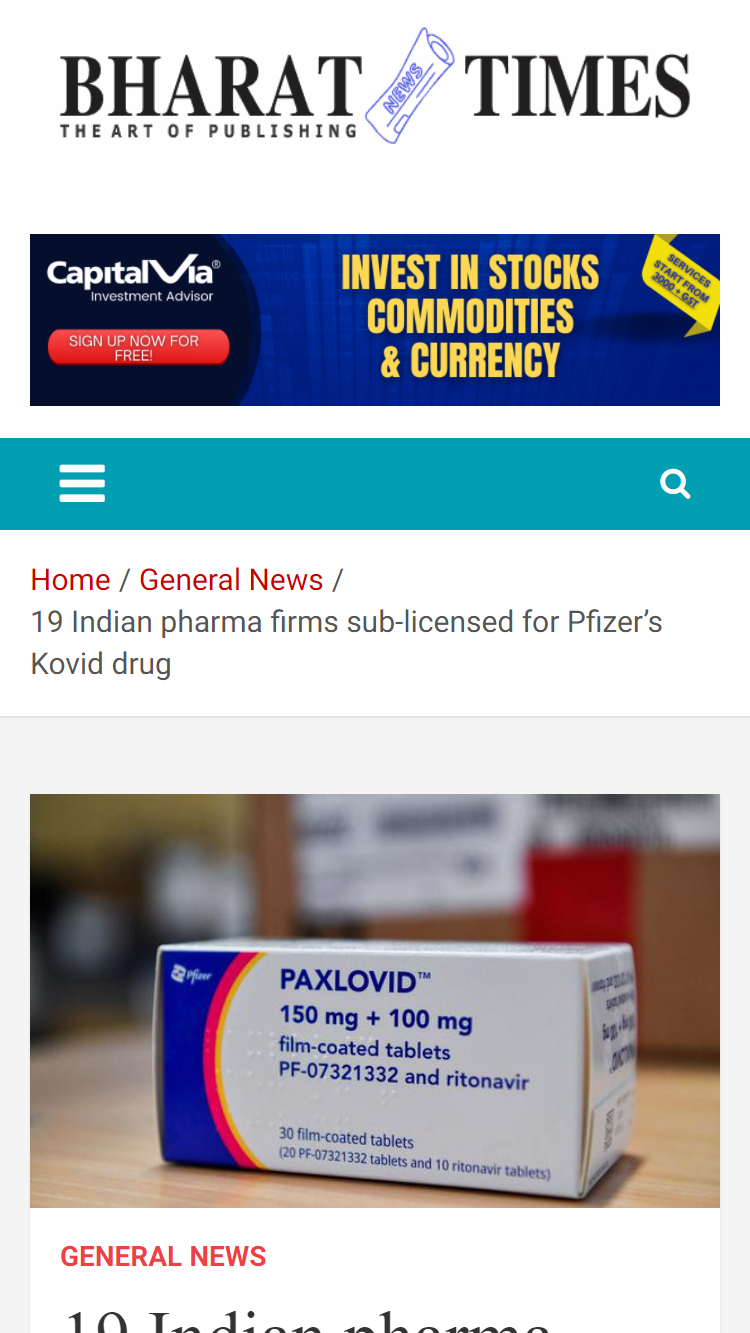
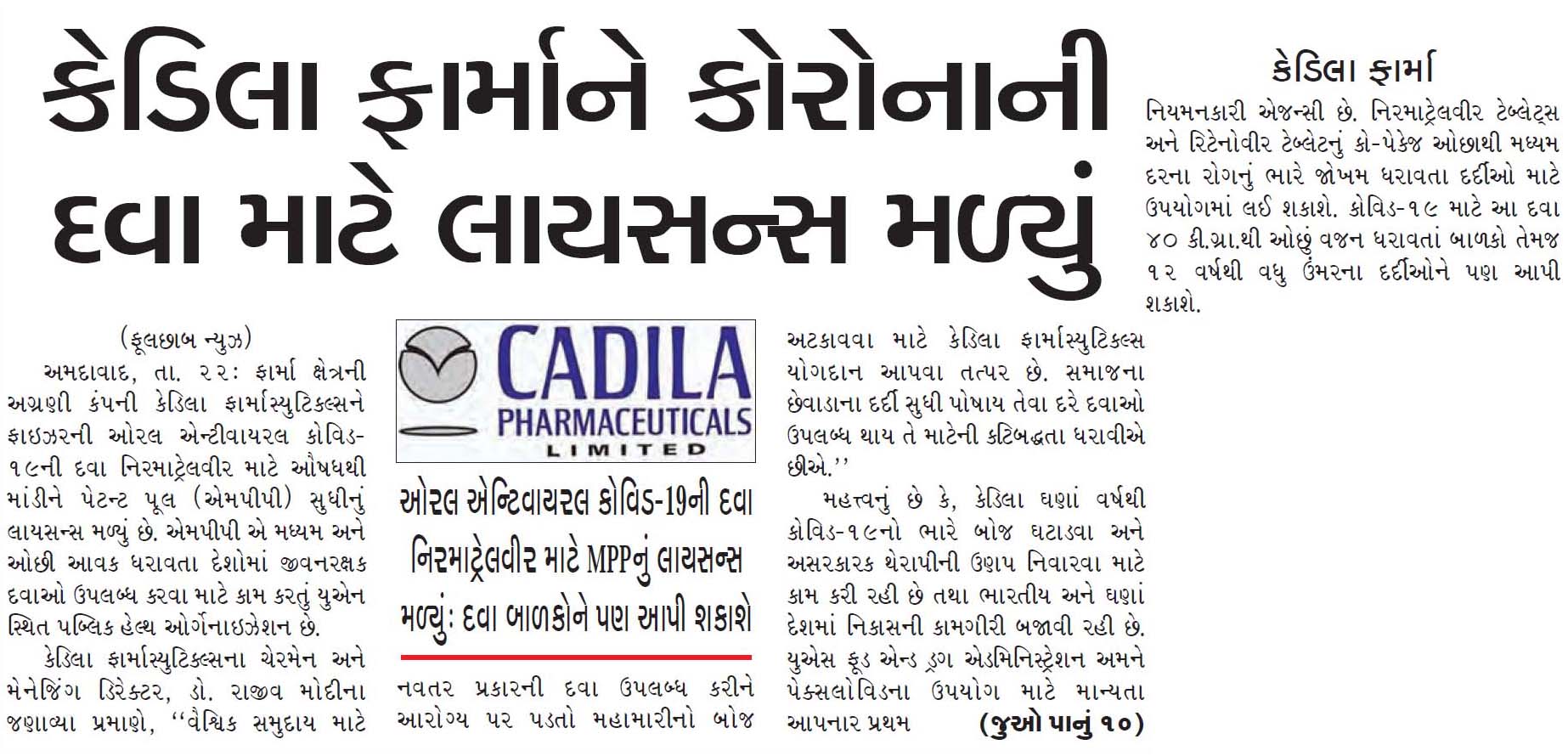
Ahmedabad, India: Pharma major, Cadila Pharmaceuticals has received a licence to manufacture a generic version of Pfizer’s oral antiviral Covid-19 medication – nirmatrelvir, through a license from Medicines Patent Pool (MPP), an UN-based public health organisation working to increase access to life-saving medicines for low- and middle-income countries.
“We are pleased to partner with MPP. to make a generic version of the innovative medicine PAXLOVID for the global community and contribute towards reducing the health burden due to the pandemic. We reiterate our commitment to make affordable innovations available to the last man in society,” said Dr Rajiv Modi, CMD, Cadila Pharmaceuticals.
Cadila will cater to Indian and export markets since many countries with high COVID 19 burden lack access to effective therapy. The U.S. Food and Drug Administration (FDA) was the first regulatory agency to authorise the use of PAXLOVID, nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use to treat high-risk mild-to-moderate Covid-19 adults and paediatric patients above 12 years of age weighing at least 40 kg.
Pfizer’s PAXLOVID™ consists of nirmatrelvir, which inhibits a SARS-CoV-2 protein to stop the virus from replicating, and ritonavir, which slows down nirmatrelvir’s breakdown to help it remain in the body for a longer period at higher concentrations. PAXLOVID is administered as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) taken together orally twice daily for five days, for 30 tablets. The drug is available by prescription only and should be initiated as soon as possible after the diagnosis of Covid-19 and within five days of symptom onset.
The FDA granted the emergency use authorisation to PAXLOVID based on clinical data from Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) trial, which enrolled non-hospitalised adults aged 18 and older with confirmed Covid-19 at increased risk of progressing to severe illness. The data showed an 89% reduction in the risk of Covid-19 related hospitalisation or death from any cause in adults treated with PAXLOVID, compared to placebo, within three days of symptom onset (primary endpoint).
Cadila Pharmaceuticals Ltd. (www.cadilapharma.com) is one of the largest privately held pharmaceutical companies in India. Over the past seven decades, Cadila has been engaged in the development of affordable medicines and making them available to patients across the world.
Array ( )
To give you the best possible experience every time you visit our site, we use cookies to identify and store your preferences on your browser. Continuing to browse our site means that you are "ok" with this. Learn more about our privacy policy.
Okay