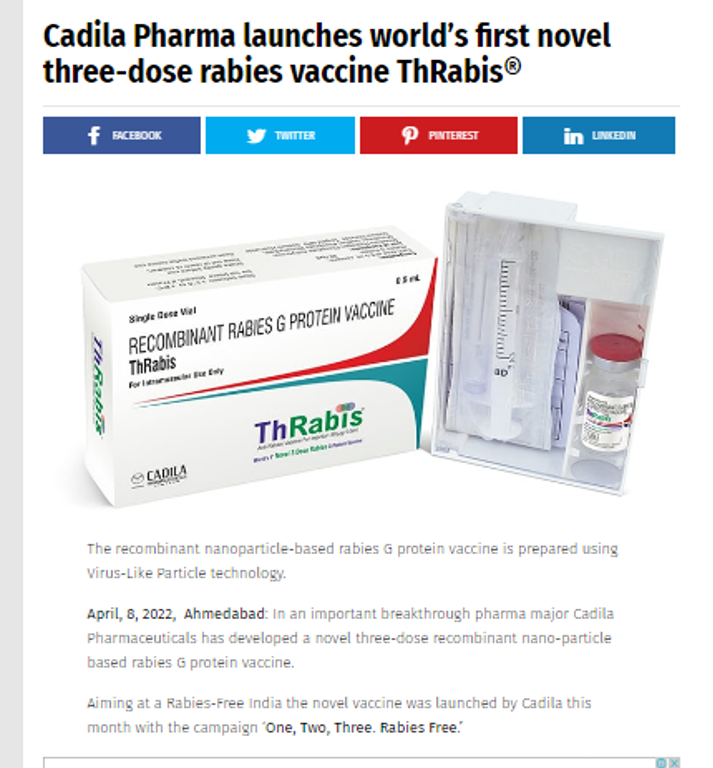
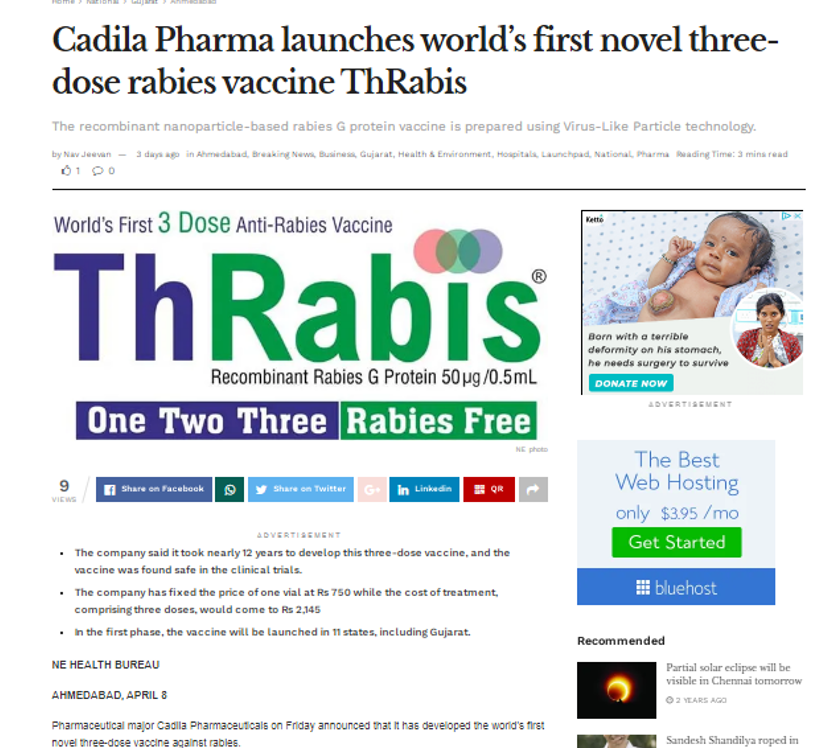
Cadila Pharma launches world’s first novel three-dose rabies vaccine ThRabis®
The recombinant nanoparticle-based rabies G protein vaccine is prepared using Virus-Like Particle technology.
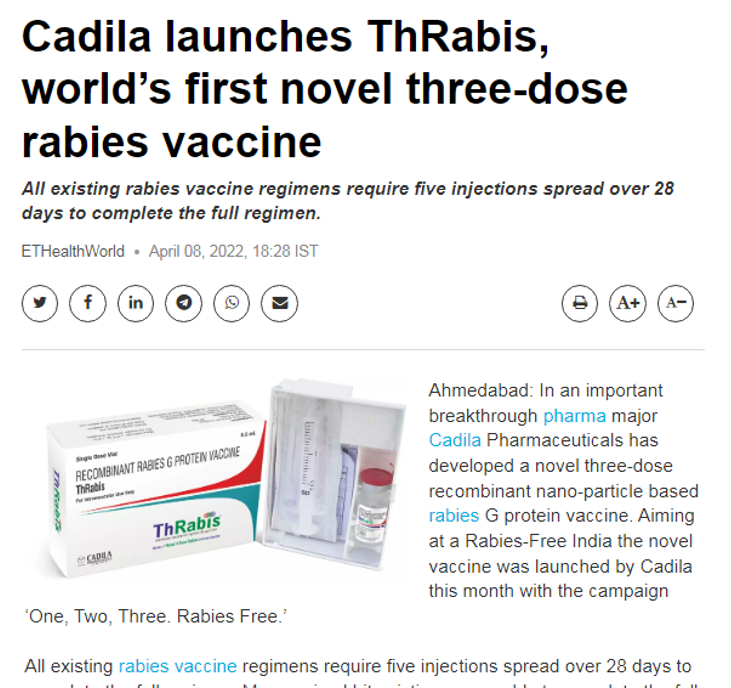
April 8,2022, Ahmedabad: In an important breakthrough pharma major Cadila Pharmaceuticals has developed a novel three-dose recombinant nano-particle based rabies G protein vaccine. Aiming at a Rabies-Free India the novel vaccine was launched by Cadila this month with the campaign ‘One, Two, Three. Rabies Free.’
All existing rabies vaccine regimens require five injections spread over 28 days to complete the full regimen. Many animal bite victims are unable to complete the full course of the vaccine due to their complicated dosing schedule, frequent visits to the hospital or clinic and potential loss of income associated with these visits. This leaves many victims unprotected and susceptible to developing rabies, which is a fatal condition.
The ‘Cadila Against Rabies’ event held in Ahmedabad was attended by key experts from the Association for the Prevention and Control of Rabies in India (APCRI) Founder President and Mentor Dr. MK Sudarshan; President Dr. DH Ashwath Narayana; Secretary General Dr. Sumit Poddar, and Treasurer Dr. HS Ravish and other leading experts. They spoke about the need to eradicate rabies and appreciated Cadila Pharmaceuticals’ efforts. The panel discussion was followed by the launch of ThRabis® – World’s 1st novel three-dose rabies vaccine.
“With this three-dose innovative vaccine, many more lives will be saved – lives that continue to be lost to a preventable disease like Rabies. This vaccine can be a game-changer in our global flight to eliminate rabies through a simpler and more convenient regimen. Continuing our journey of making affordable innovations available to the last man in society, ThRabis® is one more addition to our portfolio of indigenous innovations,” shared Dr. Rajiv Modi, CMD, Cadila Pharmaceuticals.
It is estimated that globally, rabies kills 59,000 animal bite victims every year, mainly in Asia and Africa. Over 20,000 people die in India alone, mainly because many animal bite victims do not complete the full course of the available vaccine.
About ThRabis®
The vaccine – ThRabis® is prepared by using Virus-Like Particle technology (VLP). The VLPs self-assemble from this recombinant G protein which is produced from genes cloned into baculovirus expression vectors and expressed in Spodoptera frugiperda (Sf9) insect cells. The vaccine generates antibodies against rabies G protein, which leads to virus neutralisation, as well as prevents virus attachment to the cell to confer protection against rabies.
Cadila Pharmaceuticals successfully tested immunogenicity and safety of three doses, i.e. 50 µg (microgram) on days 0, 3 and 7 of the novel vaccine in Phase-I/II and Phase-III clinical trials in healthy volunteers as well as preclinical models. The safety and immunogenicity of the vaccine were established in the trials. Besides being convenient, ThRabis® is an intramuscular vaccine and less painful for the recipients. It is also extremely convenient for doctors as it is a ready to use vaccine and does not require reconstitution prior to use (Adding water for injection and mixing it thoroughly).
“ThRabis® is a major breakthrough vaccine that can help in preventing many rabies related deaths. Unlike other approved rabies vaccines, ThRabis® is a three-dose vaccine and is administered within a week. This ensures clinical effectiveness with just three doses in seven days, and will improve compliance to complete the vaccine course,” said Mr. Suresh Gupta, President, Domestic Business, Cadila Pharmaceuticals.
About Cadila Pharmaceuticals
Cadila Pharmaceuticals Ltd. (www.cadilapharma.com) is one of the largest privately-held pharmaceutical companies in India. Over the past seven decades, Cadila Pharmaceuticals has been engaged in the development and manufacturing of affordable medicines and in making them available for patients across the world. Its innovation-driven drug discovery processes ensure the health and well-being of people around the world.