Pharmacovigilance is like a health detective, watching out for any issues with medications and vaccines.
If you experience any side effect, let us know – together, we can keep healthcare safe and effective!
Your Safety, Our Priority!
Help Us, Help You!
Think of pharmacovigilance as a safety net for your medicine and vaccines.
We’re on a mission to catch any unexpected side effects or issues before they become big problems.
So, Share your experiences!
Your Feedback Fuels Safety!
Report a strange reaction to a medication or vaccine, help us to protect millions.
Let’s work together to keep treatment safe and sound!
Who should Report ?
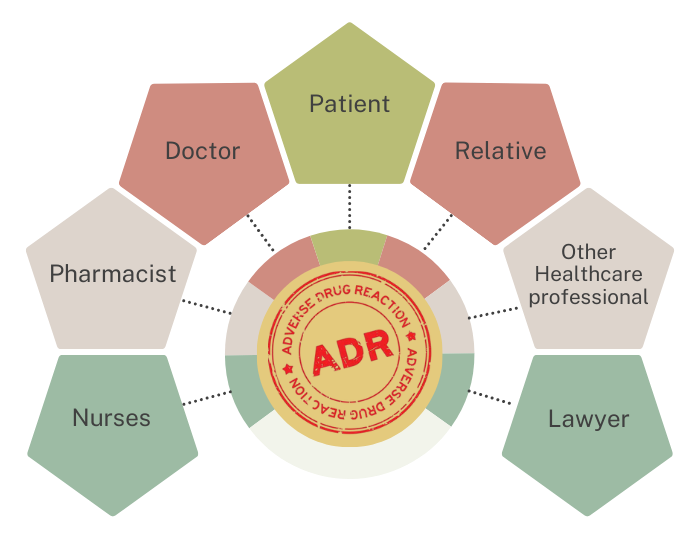
When to Report ?

Report for Cadila Pharmaceuticals Limited:
- All suspected adverse drug reactions (Side effects)
-
- Serious reactions include those which are:
-
-
-
-
-
- Fatal (death)
- Life-threatening
- Disabling or incapacitating
- Result in or prolong hospitalization
- Congenital abnormalities or Medically significant
- Other side effects
- Product Quality related complaints.
-
-
-
-
Related to identity, purity, quality, strength, efficacy, safety, physical changes, labelling defects packaging, storage, handling of product or any other defects
Important: Fill and send the initial Info on Adverse Drug Reactions (ADR) report form within 24 hours of receipt
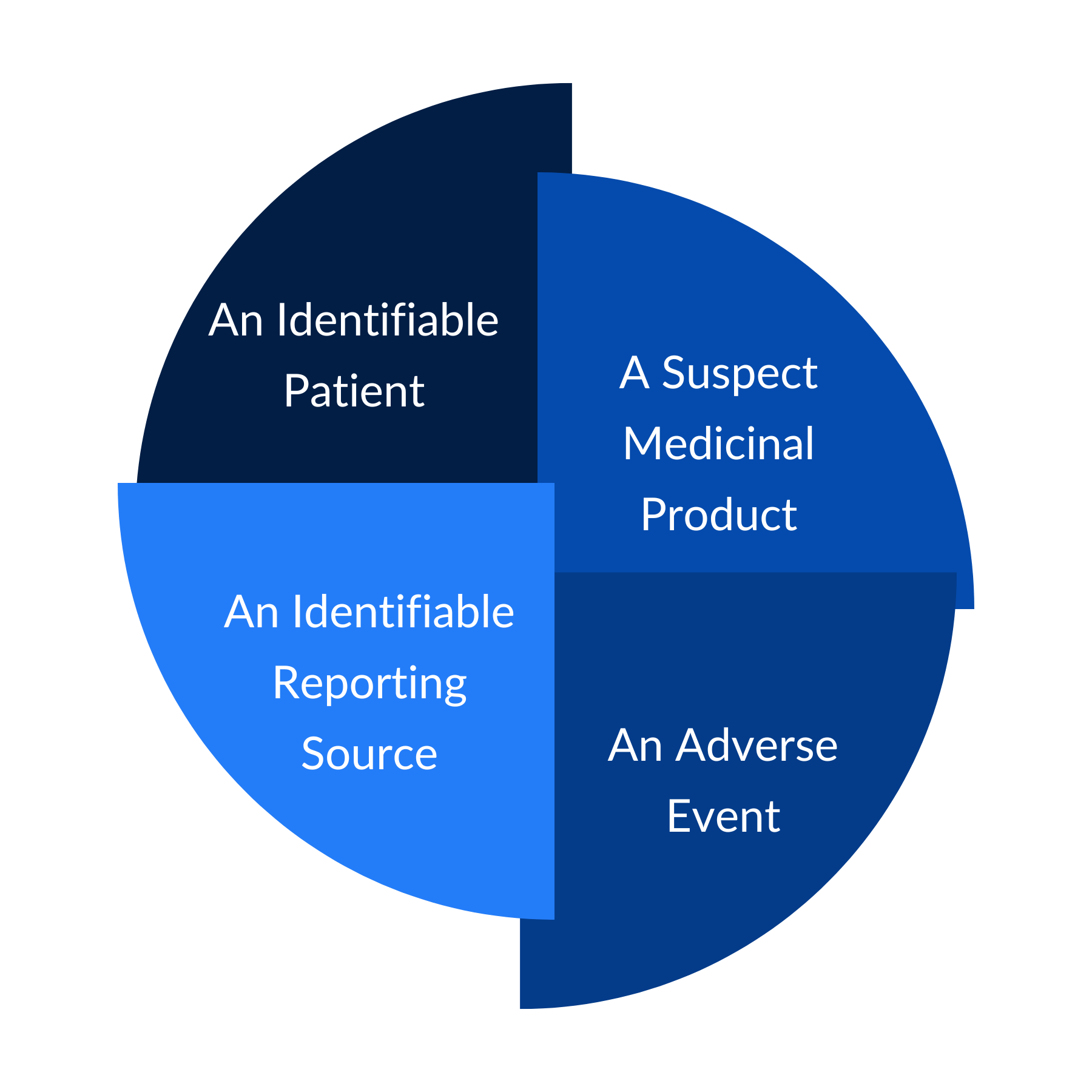
What to Report?
- Patient Information
- Age, Gender, Other disease or on other medication
- Reporter information
- Name, Contact Number, Address
- Drug Information
- Drug Name (Brand name, Generic Name); Batch Number, Manufacturing and Expiry Date
- Event description
- Name of Event, Date of Event, Outcome, Action taken
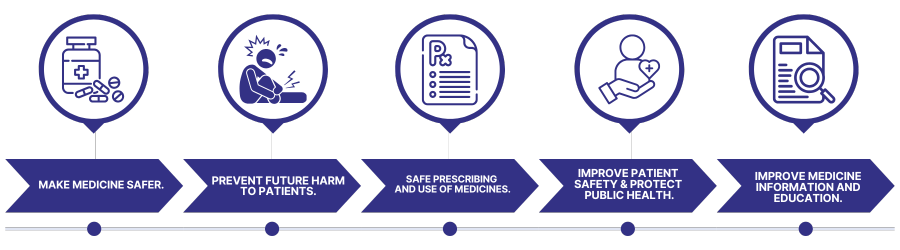
Why to Report?
Where to Report?
Patient can report suspected side effects:
📧 Email: safety.cadila.global@cadilapharma.co.in
📞 Toll free Number: 18005325326
📞 Landline Number: +91-2718-251334
Product Information
India (vaccine)
United Kingdom
I, Commit to report all adverse reactions to Cadila Toll Free Helpline.
I commit myself to be vigilant and support patient safety.

