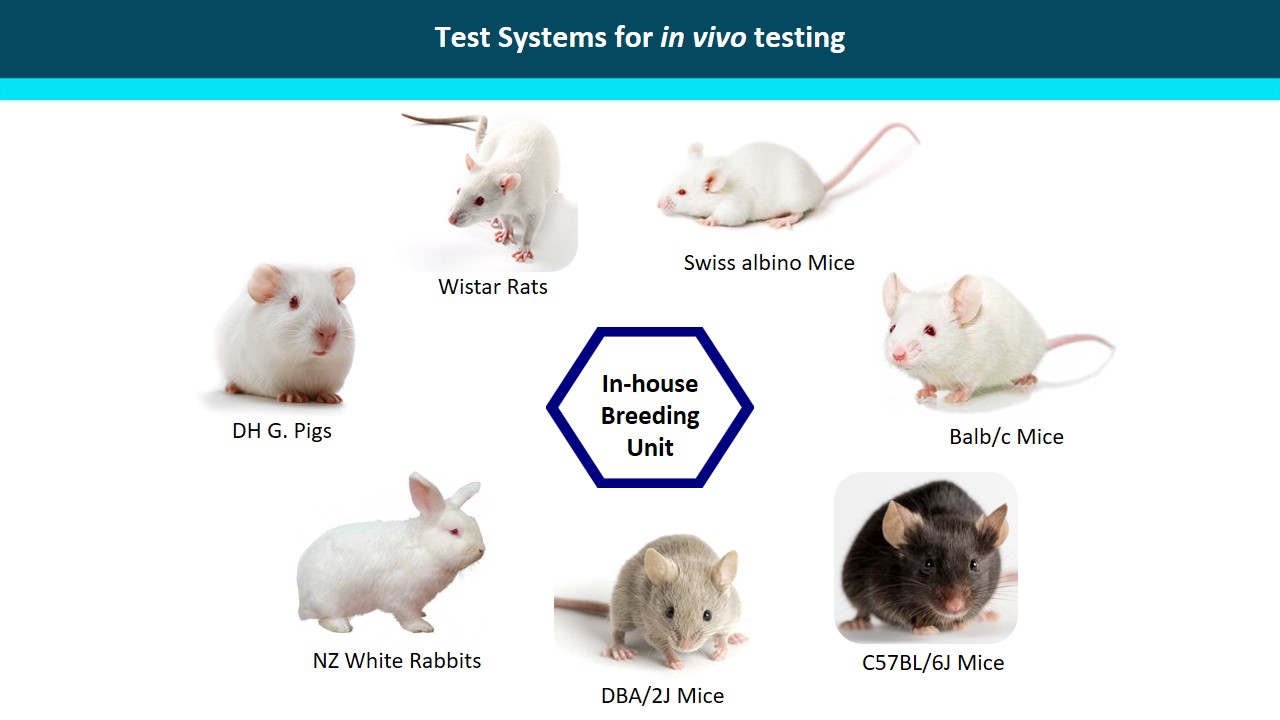
We offer regulatory solutions related to non-clinical testing of pharmaceutical/biotechnology products, vaccines, cosmetic products, veterinary drugs as well as food additives, feed additives, industrial chemicals and medical devices.
Our CRO also offers an end-to-end solution for drug development programs i.e., from NCE synthesis to clinical trials. Additionally, our CRO provides a full range of services for drug development projects, including NCE synthesis to clinical trials.
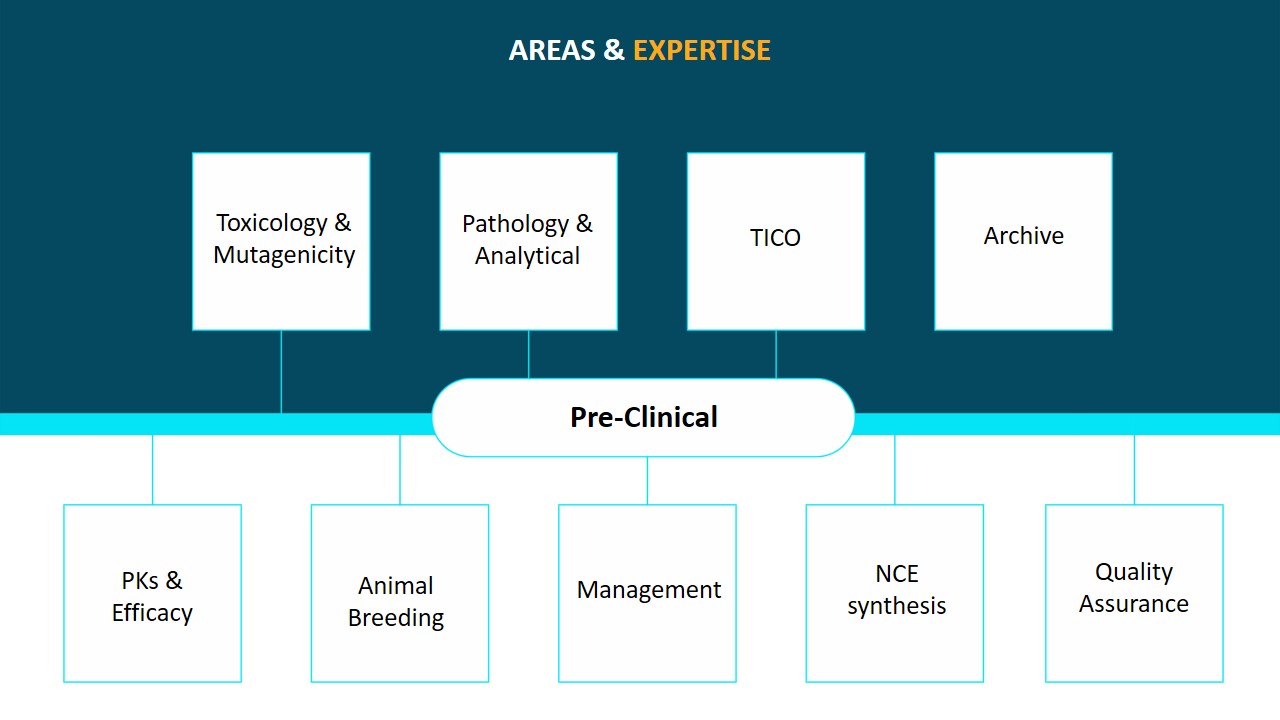
We offer expertise in a variety of areas, including APIs and formulations, recombinant and gene therapy products and vaccines, herbal remedies, nutraceuticals, medical devices, and the chemical and agrochemical industries. We have conducted various collaborative IND filing projects for Cadila
Pharmaceuticals Ltd and its biotechnology division. Additionally, we have conducted research with leading institutions, mainly in the United States and Europe.
Key Highlights:
- Widespread research partners across the globe
- >5 IND submissions
- Successful dossier submission by the sponsor in USFDA for first in world vaccin
- Audited by various regulatory/competent authorities:
- NGCMA, Govt. of India for compliance of OECD Principles of GLP
- CPCSEA inspectors
- State FDA
- ISO
- Sponsor / professional auditors
- The Public Testing Laboratory (PTL) of the CRO is approved (licensed) by Food & Drug
Control Administration (FDCA), Gujarat for carrying out biological Pharmacopoeia
test/batch release tests on various products.