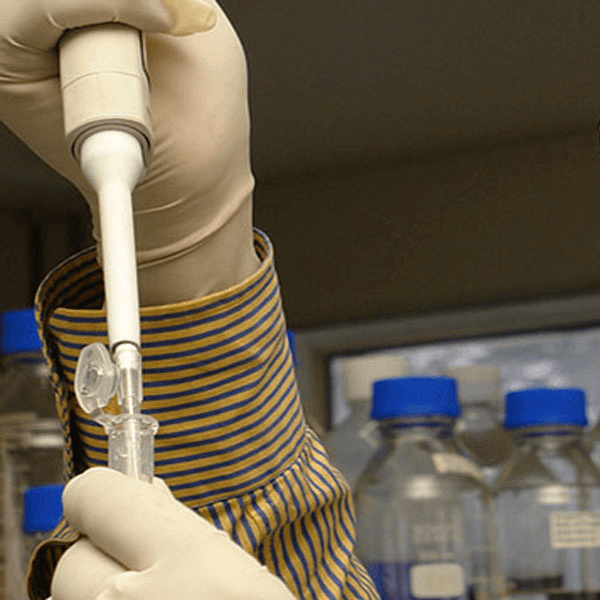
At the Forefront of the Innovative Formulation
Like our extensive range of finished products, our formulation R&D team caters to every development need for domestic, regulated and semi-regulated markets. Our scientists undertake novel molecules from drug discovery to all the way upto clinical development phases by determining its composition, correct dosage, safety and efficacy for manufacturing and commercialization. Besides developing formulations, our R&D team improves existing formulations to ensure maximum efficacy and product compliance by the patient.
Besides developing formulations for potential new chemical entities, we also work to improving existing Cadila formulations. Our aim is to ensure maximum efficacy for an existing drug, as well as increasing the chances of product compliance by the patient. Developing new ways of administering old drugs to patients often calls for sophisticated knowledge of chemistry, as well as, a sharp understanding and experience of patient behaviour and various compliance factors.
Towards this end, our efforts are consistently targeted at making the delivery of new, as well as old drugs more efficient in order to ensure maximum relief for patients and limit the potential side-effects of any drug.
In the end, Cadila believes that formulation is a data-driven science that must adhere to a laid-down, quality-vetted process. After carefully analysing and understanding a client's specific needs, we create a target product profile (TPP) that is clearly and unambiguously defined before developing any product algorithm.
All these years, we have been very active at devising elaborate strategies for formulations that have been very effective at meeting stringent international quality norms and regulatory practices. This is how we have managed to come up with a huge line of affordable products that also ensure patient compliance.
Cadila has in place a Quality by Design (QBD) approach that uses time-tested Modelling and Design of Experiments (DoE) approach to deliver a strong regulatory package on a product.
Leading from the front, we know from our past experience that in research, no single approach can effectively solve all problems. Therefore, our strategy combines the application of multiple technologies in our drug formulation process.
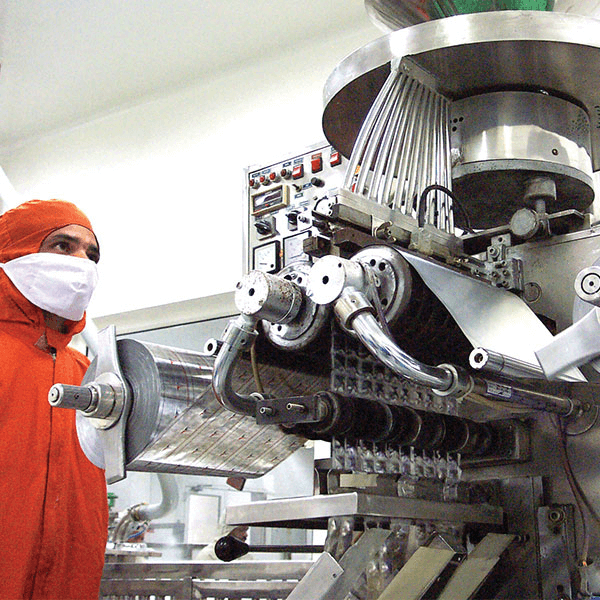
Our Development Process
Cadila believes that formulation is a data-driven science that must adhere to a laid-down, quality-vetted process. After carefully analysing and understanding a client's specific needs, we create a target product profile (TPP) that is clearly and unambiguously defined before developing any product algorithm.
In addition, our strategies for formulations development ensure compliance with stringent international quality norms and regulatory practices. Our Quality by Design (QBD) approach uses time-tested Modelling and Design of Experiments (DoE) approach to deliver a robust regulatory package of a product.
Array ( )
To give you the best possible experience every time you visit our site, we use cookies to identify and store your preferences on your browser. Continuing to browse our site means that you are "ok" with this. Learn more about our privacy policy.
Okay

